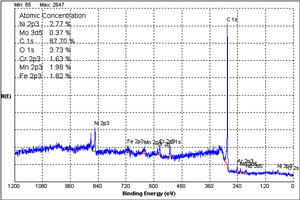
In XPS, a monochromatic x-ray is employed to eject electrons from the atoms in a solid sample. The kinetic energy of the ejected electron is measured by an electrostatic analyzer that focuses the emitted electrons onto a solid state detector. This is achieved in ultrahigh vacuum. Based on the atomic structure of the emitting atom, the binding energies of the emitted electrons are quantized. The exciting x-ray penetrates deeply into the sample and excites photoelectrons throughout the penetration depth. However, electrons emitted from deeply in the solid lose their energy through collisions with the atomic lattice. Therefore, only electrons emitted from the first several atomic layers of the solid arrive at the detector without losing energy. Thus, XPS is truly a surface technique. XPS is sensitive to all elements in the periodic chart with the exception of hydrogen and helium. The core electron binding energies measured undergo chemical shifts as a function of the oxidation state of the emitting atom. Thus the chemical state of atoms present on the surface of the solid can be determined. The intensity of the photoelectron line is proportional to the chemical composition of the surface. Therefore, semi-quantitative analysis of the surface is readily available. Both conducting and insulating samples can be analyzed.