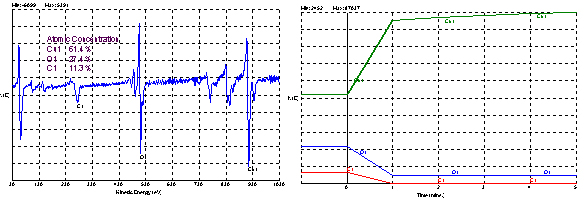
AES is closely related to XPS. In Auger electron spectroscopy, electrons are ejected from a solid by an energetic electron beam. Once the core electron is ejected, conservation of energy mandates that an electron from a higher atomic orbital descends to fill the lower vacancy with the subsequent emission of a secondary Auger electron. The Auger electron is then focused by an electrostatic analyzer onto a solid state detector. The energy of the Auger electron is quantized by the atomic structure of the emitting atom. Only those electrons emitted from the first several atomic layers make it to the detector without losing energy through collisions with the lattice. Because of the exciting electron beam and the high level of electron noise in the analysis chamber, Auger spectra are typically recorded in derivative mode. Auger energies are not useful for determining the chemical states of surface atoms. Line intensities are proportional to atomic composition so semi-quantitative analysis is possible. AES is an ultra-high vacuum technique. Typically, only conducting samples are analyzed by Auger electron spectroscopy.